 SynLube™ Lube‑4‑Life®
SynLube™ Lube‑4‑Life®
 SynLube™ Lube‑4‑Life®
SynLube™ Lube‑4‑Life®
By
Professor D. H. Everett
School of Colloid Science
University of Bristol
Bristol, England
Colloid science is experiencing a renaissance. The beginnings of this new phase can be traced back to 1930's when a scientific understanding of at least some colloidal phenomena began to evolve.
Since then activity has increased steadily. Fundamental knowledge has developed rapidly and the resulting insights have been exploited extensively in industry. Much empiricism, which for generations have guided practical applications, has gradually been shown to have origin in the laws of physics and chemistry.
There is still much to be learned, but a stage is being reached at which it is becoming possible to present a general account of the main themes of colloid science in terms of basic physical chemistry.
It has also become clear that there is a need for a short introduction that presents an outline of colloid science starting from a relatively elementary knowledge of science.
This booklet will provide an introduction to colloids for those with a basic familiarity with physical chemistry and serve as a jumping-off point for those wishing to go more deeply into the many fascinating areas, which constitute the broad range of fundamental and applied colloid science.
One of the major features of the development of colloid science has been the impressive advances that have been made in colloid technology. However, although many large companies have exploited recent developments, it is unfortunately the case that smaller companies have often failed to recognize the potential applications of colloid science in enhancing the efficiency of their processes or the quality and range of their products. Attention is therefore drawn to some of those areas of the subject that have been, or can be, of industrial importance. In a booklet of this size it is impossible to deal in detail with such matters.
Thus the booklet as a whole may prove of value both to those entering industry with little previous knowledge of colloids and to those already in industry wishing to become familiar with recent ideas.
However, the major motivation for this booklet was to explain the concept of colloids to customers and distributors of SynLube™ Lube‑4‑Life® products, which are based on synthetic colloidal technology (Syn-Sol).
"To some the word 'colloidal' conjures up visions of things indefinite in shape, indefinite in chemical composition and physical properties, fickle in chemical deportment, things infilterable and generally unmanageable."
(Hedges, 1931)
The above remarks reflect the impression created by many textbooks of physical chemistry, if they begin to mention colloids at all.
In fact, in both its experimental and theoretical aspects, and no less important in its technological applications and in the appreciation of its biological implications, colloid science has made impressive progress in the last few decades.
In the following pages attempt is made to briefly summarize the basic concepts of colloid science and to dispel some of the doubts expressed in the above quotation.
A full understanding of the properties of colloids calls upon a wide range of physical and chemical ideas, while the multitude of colloidal systems presented to us in nature, and familiar in modern society, exhibit a daunting complexity.
It is this that has delayed the development of colloid science, since a detailed and fundamental theoretical understanding of colloidal behavior is possible only through a thorough knowledge of broad areas of physics, chemistry, and mathematical physics, together in many instances with an understanding of biological structures and processes.
On the experimental side there is an ever-increasing emphasis on the application of modern physical techniques to colloidal problems. Colloid science is thus a truly interdisciplinary subject.
Nevertheless, despite the sophistication needed for the development of a complete quantitative theory of colloids, the basic principles that underlie many colloid problems can be seen as extensions to such systems of the fundamental concepts of physical chemistry.
One important objective of this booklet is to emphasize the close link between colloid science and physical chemistry and to show how a broad understanding can be built up on a few relatively simple physic-chemical ideas. We shall not only seek common features revealed by experimental study but also, of much greater significance, try to identify the fundamental concepts that link together many apparently unconnected aspects of the subject.
In setting out to define the scope of colloid science, it should first be said that any attempt to lay down too rigid a scheme of definitions and nomenclature is likely to be unnecessarily restrictive.
Rather than try at the outset to develop a formal definition, it is preferable to describe examples of systems to which the term `colloidal' is now applied.
The etymology of the term colloidal (glue-like) introduced by Thomas Graham is now largely irrelevant.
An essential part of any study of physics and chemistry involves first the recognition of three states of matter (solid, liquid, and gas) and a general discussion of the transformations (melting, sublimation and evaporation) between them.
Pure substances are considered, and then attention passes to solutions, which are homogeneous mixtures of chemical species dispersed on a molecular scale. What remained largely unrecognized until about a century and a half ago was that there is an intermediate class of materials lying between bulk and molecularly dispersed systems, in which, although one component is finely dispersed in another, the degree of subdivision does not approach that in simple molecular mixtures.
Systems of this kind, colloids, have special properties which are of great practical importance, and they were appropriately described by Ostwald as lying in the World of Neglected Dimensions.
They consist of a dispersed phase (or discontinuous phase) distributed uniformly in a finely divided state in a dispersion medium (or continuous phase).
As familiar examples of colloidal systems we cite the following:
In association colloids molecules of soap or other surface-active substances are associated together to form small aggregates (micelles) in water. The aggregates formed by certain substances may adopt an ordered structure and form liquid crystals. Many biological structures are colloidal in nature.
For example, blood is a dispersion of corpuscles in serum, and bone is essentially a dispersion of a calcium phosphate embedded in collagen.
In the above examples, which may be called simple colloids, a clear distinction can be made between the disperse phase and the dispersion medium. However, in network colloids this is hardly possible since both phases consist of interpenetrating networks, the elements of each being of colloidal dimensions.
Porous solids, in which gas and solid networks interpenetrate, two-phase glasses (opal glasses) and many gels are examples of this category.
Furthermore, there are other instances (multiple colloids) that may involve the co-existence of three phases of which two (and sometimes three) phases are finely divided.
One example is a porous solid partially filled with condensed vapor, when both the liquid and vapor phases within the pores are present in a finely divided form.
Similar situation arises when oil and water co-exist in the pores of an oil-bearing rock, also in frost heaving when water and ice co-exist in a porous medium.
Multiple emulsions consist for example of finely divided droplets of an aqueous phase contained within oil droplets, which themselves are dispersed in an aqueous medium.
Some of the more important types of colloidal systems outlined above are
summarized in:
Table 1.1 below.
| Examples | Class | Disperse phase | Dispersion medium |
|---|---|---|---|
| Mist Tobacco smoke "aerosol" sprays |
Liquid aerosol or Aerosol of liquid particles |
Liquid | Gas |
| Industrial smokes | Solid aerosol or Aerosol of solid particles |
Solid | Gas |
| Milk Butter Mayonnaise Asphalt Pharmaceutical creams |
Emulsions | Liquid | Liquid |
| Inorganic Colloids silver iodide metallic hydroxides paints SynLube™ lubricants |
Colloidal suspensions or "Sols" | Solid | Liquid |
| Clay slurries Toothpaste Muds Polymer lattices |
When very concentrated called "paste" | Solid | Liquid |
| Opal Pearl Stained glass Pigmented plastics |
Solid suspension or dispersion | Solid | Solid |
| Froths Foams |
Foam | Gas | Liquid |
| Meerschaum Styrofoam Expanded plastics |
Solid foam | Gas | Solid |
| Macromolecular colloids Jellies Glue |
Gels | Macromolecule | Solvent |
| Association colloids Soap/Water Detergent/Water Dye solutions |
Micelles | Solvent | |
| Biocolloids Blood Bone |
Corpuscles Hydroxyapatite |
Serum Collagen |
For simplicity we shall limit ourselves in this booklet to a discussion of simple colloids, although the ideas developed can be extended and applied to more complex systems.
The fundamental question, which has to be answered, is: 'What do we mean by "finely divided"?'
It turns out, for reasons which will soon be apparent, that systems usually
exhibit properties of a specifically "colloidal
character" (which we shall explain in more detail later) when the
dimensions of the dispersed phase lie in the range of 1 to 1000 nm
(nano-meters),
i.e. between 10 Ã (angstrom) and 1 µm (micron).
These dimensions are below the limits of resolution of simple optical microscopes so that direct imaging and measurement of the sizes of colloidal particles only became possible with the development of electron microscopes.
These dimensional limits are not rigid, for in some special cases (e.g. emulsions and some slurries) particles of much larger size are present.
Moreover, it is not necessary for all three dimensions to lie below 1 µm, since colloidal behavior is observed in systems containing fibers in which only two dimensions are in the colloid range.
In other systems, such as clays and thin films, only one dimension is in the colloid range.
This is illustrated schematically in Figures 1.1 and 1.2, while Figure 1.3 shows electron microscope photographs of colloidal particles of several types.
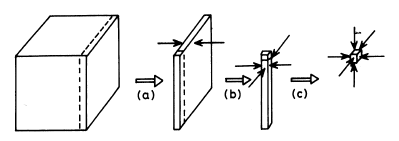
Figure 1.1
Schematic representation of the subdivision of a cube to give colloidal systems of different kinds:
(Adapted from A. von Buzagh, "Colloid Systems", Technical Press, London, 1937)
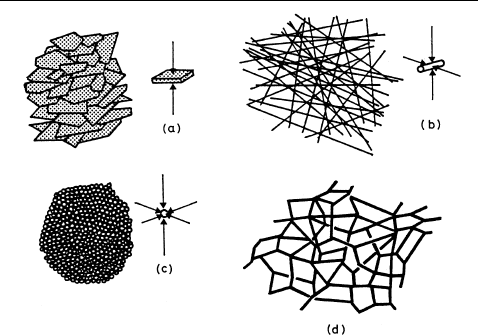
Figure 1.2
Schematic representation of colloid systems of various types:
(By Professor D. H. Everett, School of Colloid Science, University of Bristol, October 1987)
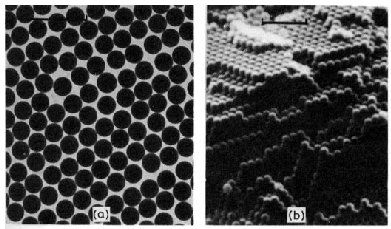
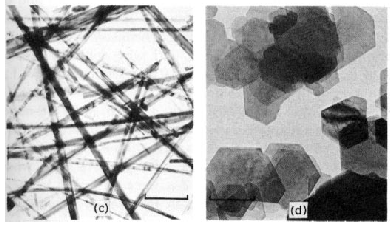
Figure 1.3
Electron micrographs of colloidal materials in which three, two, and one
dimensions lie in the colloid range
(bars indicate 1 µm = micron)
(By Dr. D. W. Thompson, School of Chemistry, University of Bristol)
Colloids in which the particle size is below about 10 nm often require special consideration.
One example of such particles are the nuclei which initiate bulk phase changes. The justification for including macromolecular solutions and association colloids within this classification arises from the fact that the particles within them are either macromolecules of considerable length, which even when coiled up have diameters of well over 1 nm, or aggregates of smaller molecules forming micelles of a size falling within the colloid size range.
Biocolloids again have their individual characteristics, but once more the presence of structures of colloidal dimensions justifies their inclusion as examples of colloids.
The limit below which colloid behavior merges into that of molecular solutions is usually presumed to be around 1 nm (10 Ã).
An alternative subdivision of colloids which has been widely used in the past is into:
Colloids, depending on whether the particles can be described in the former
case as
"solvent hating" or in the latter case as "solvent loving".
These characteristics are deduced from the conditions required to produce these colloids and from the means available for their redispersion after flocculation or coagulation.
It will become apparent later that, while this subdivision has many useful aspects, it is neither entirely logical nor sufficiently all embracing, and we shall make only limited use of it.
Because of the range of dimensions involved in colloidal structures, the surface-to-volume ratio is high and a significant proportion of the molecules in such systems lie within or close to the region of inhomogeneity associated with particle/medium interfaces.
These molecules will have properties (e.g. energy, molecular conformation) different from those in the bulk phases more distant from the interface.
It is then no longer possible (as we do in bulk thermodynamics) to describe the whole system simply in terms of the sum of the contributions from the molecules in the bulk phases, calculated as though both phases had the same properties as they have in the bulk state.
A significant and often dominating contribution comes from the molecules in the interfacial region.
This is why surface chemistry plays such an important part in colloid science and why colloidal properties begin to become evident when the particle size falls below 1 µm.
We can see this in the following way:
The surface area associated with a given mass of material subdivided into equal-size particles increases in inverse proportion to the linear dimensions of the particles.
Thus the area exposed by unit mass (the specific surface area, as ) is given by 6/ρd.
Where ρ is the density of the material and d is the edge length in the case of cubic particles or the diameter in the case of spheres.
If the material is made up of molecules of linear dimension h and molecular volume ~h3 , then the fraction of molecules in the surface layer is given approximately by 6(h/d).
Thus for a substance of molar volume 30 cm3 mol -1 or
of molecular volume 0.05 nm3 (e.g. silver bromide)
h = 0.37 nm.
For a 1-cm cube only two or three molecules in ten million are surface molecules, and these have a negligible influence on its properties.
However, when divided into 1012 particles of 1 μm, one molecule in four hundred and fifty is a surface molecule, and the properties of the system begin to be affected.
At 10 nm the ratio rises to nearly one in four and surface effects became dominant.
Beyond this it is hardly possible to decide what we mean by a surface molecule and, as indicated above, special considerations apply to the size range 1-10 nm.
To illustrate this point, Figure 1.4 on next page shows the variation of the percentage of surface molecules with particle size for the typical case of silver bromide.
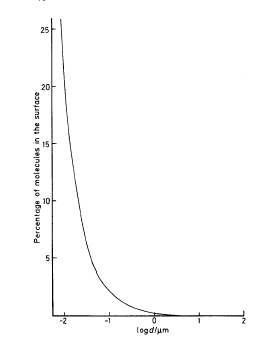
Figure 1.4
Variation of the percentage of molecules in the surface as a function of particle size for a substance with a molar volume of 30 cm3 mol-1
This approach to colloids, emphasizing the importance of surface or interfacial properties, suggests a more meaningful description of colloids as microheterogeneous systems, the microheterogeneity being characterized by lengths in the range 1-1000 nm.
It should be noted, however, that some typically colloidal phenomena, such as light scattering, are exhibited (though very weakly) by systems in which the microheterogeneity arises from random kinetic fluctuations in density in an otherwise uniform system of small molecules such as a gas or a liquid, while in some cases (e.g. suspensions of relatively coarse solid particles) certain colloid-like properties may persist to particle sizes much larger than the above maximum.
Before proceeding further, it will be helpful to introduce a number of additional terms, which are widely used in the description of colloidal behavior.
Disperse systems in which all the particles are of (approximately) the same size are said to be monodisperse (or isodisperse); conversely, if a range of particle sizes is present, they are polydisperse.
In certain circumstances, to be discussed in greater detail later, the particles of a dispersion may adhere to one another and form aggregates of successively increasing size which may, despite the tendency of thermal motion to keep them in suspension, separate out under the influence of gravity.
The nature of the aggregated material may depend on the conditions of its formation, or it may change with time.
An initially formed, rather open aggregate is called a floc and the process of its formation flocculation. The floc may or may not separate out.
If the aggregate changes to, or is produced in, a much denser form, it is said to undergo coagulation with the formation of a coagulum. An aggregate usually separates out either by sedimentation (if it is more dense than the medium) or by creaming (if it is less dense than the medium).
Since in many cases it is not readily apparent which type of aggregate is formed, the terms flocculation and coagulation have often been used interchangeably, but the more specific meanings introduced above are gaining more general acceptance.
One characteristic that is sometimes used to infer a distinction between the two is whether aggregation is reversible. It is usually supposed that coagulation is irreversible whereas flocculation can be reversed in the process of deflocculation.
However, conditions can sometimes be found under which even coagulated systems can be redispersed.
The meaning of the term stability as applied to colloid systems is to be considered both for the conditions of initial dispersion, floc or coagulum and redispersion.
Although the true nature of colloids was not appreciated until relatively recently, Man has observed and made use of colloidal systems and their properties since the earliest days of civilization.
Moreover, colloids have played in geological time, and play today, an important role in many natural phenomena.
Perhaps the oldest record of a colloidal phenomenon is that of the deposition of silt at river mouths mentioned in the Babylonian Creation myth which, incidentally, was inscribed on tablets of clay, themselves an example of a colloidal material.
The Book of Genesis refers to clouds and the fall of rain.
But early Man must also have been familiar with many other colloidal phenomena, such as the effect of walking on wet sand and the treachery of quicksand.
He soon exploited them in the preparation of butter, cheese, and yogurt and in the making of bread.
His early technology, too, often depended on colloids and their properties:
Indeed, there can have been few aspects of his domestic life that were independent of the behavior of colloids, either of natural occurrences or prepared by him.
The same is even truer today: the list of colloids and colloidal processes of vital importance to modern living and industrial technology is almost unlimited.
Their diversity can be appreciated by quoting just a few examples, which include the following:
The difference today is that colloid technology is rapidly becoming more rational and scientifically based and is leaving behind many of the empiricisms that characterized those earlier crafts which depended on controlling and using colloidal materials.
Despite this long history, the scientific study of colloids is a relatively recent development.
It is true that the alchemists prepared and used two important forms of colloidal gold, namely potable gold (supposedly the Elixir of Life) and Purple of Cassius, used to make ruby glass.
Macquer in his Dictionary of Chemistry (1774) speculated that in these, gold was present in a finely divided form.
But the first experimental studies date from the early years of the nineteenth century, when Selmi (1845) prepared what were then called demulsions of sulfur and silver halides.
It was not until 1856 that Michael Faraday made the first systematic study of colloidal gold, which will be outlined in the following section, and put forward ideas which can still be seen in modern theories concerning the factors responsible for the stability of these dispersions.
The word `colloid' was coined later by Thomas Graham in 1861 to describe systems, which exhibited slow rates of diffusion through a porous membrane, of which glue solutions were a typical example.
The usefulness of this definition depends on the significant decrease in the diffusion rate when the size of the diffusing particle exceeds a few nanometers. However, such solutions are but one example of the wide class of disperse systems described above, although this was not appreciated immediately.
The slow progress in the understanding of colloidal behavior compared with that in other branches of chemistry and physics was in large part due to the extreme difficulty of preparing well-characterized materials with reproducible properties. As exemplified by the quotation heading this section, and in part to the absence of adequate theoretical knowledge to provide a basis for understanding the factors controlling these properties.
Recent progress has followed the reduction and, in some cases, the elimination of these barriers.
Thus methods of preparing well-characterized colloids have made it possible to perform quantitative and reproducible experiments, while the development of theories of intermolecular forces, electrolyte solutions, and polymers was essential before the concepts they introduced could be brought together and applied in colloid science.
Coupled with these factors, and playing an increasingly important role, has been the application to colloids of sophisticated modern instrumental techniques, including:
The guidance provided by basic research into the fundamental factors controlling colloidal behavior has proved, and is increasingly proving, of immense value in enhancing industrial processes that involve colloids and in developing new processes and products.
Among the many examples, which have taken place in the last, twenty to forty years are:
These are based in large measure on an increased understanding of the principles of colloid and surface chemistry.
Antonino Tabascio first initiated the marriage of colloid chemistry and Tribology in 1944 and SynLube™ Lube‑4‑Life® lubricants are direct result of his Syn-Sol experiments.
It so happens that one of the first colloidal dispersions to have been examined systematically will suit our purpose admirably.
During 1856-7 Michael Faraday first prepared colloidal gold by reducing an aqueous solution of gold chloride with phosphorus to yield a ruby-colored liquid.
He showed by chemical tests that the gold was no longer present in an ionic form but that reagents that dissolve metallic gold were able to remove the color. He concluded that the gold was dispersed in the liquid in a very finely divided form, the presence of which could be detected by the bluefish opalescence observed when a narrow, intense beam of light is passed through the liquid.
This phenomenon was subsequently investigated by Tyndall and is known as the Tyndall effect.
Faraday observed that the addition of a small amount of various salts changed the color from ruby towards blue and that the blue liquid tended to deposit solid.
Neither the blue liquid nor these deposits could be changed back to ruby.
Faraday found that the gold sol could also be produced in the presence of a warm gelatin solution, which on cooling set to a jelly. Moreover, when prepared in this way addition of salt to the warm solution did not change the color to blue.
Faraday concluded that the change from ruby to blue resulted from an increase in particle size.
Of the particles in the ruby liquid he said: "Whether the particles be considered as mutually repulsive, or else as molecules** of gold with associated envelopes of water, they differ from those particles which by the application of salt or other substances are rendered mutually adhesive, and so fall and clot together.'"
(** Note that the modern use of the word "molecule" was not introduced until Cannizarro publicized the work of Avogadro in 1859)
His observations on the jellied samples implied, he believed: "a like association (of the gold particles) with that animal substance", which explained their stability in the ruby form.
In this series of experiments Faraday thus demonstrated some of the more important properties of colloidal dispersions: light scattering, sedimentation, coagulation by salts, and their `protection' from the effects of salt by gelatin. His interpretation of these observations was remarkably perceptive, in contrast to the speculations of some of his contemporaries. He correctly surmised that the change induced by changing conditions "is not a change of the gold as gold, but rather a change in the relations of the surface of the particle to the surrounding medium".
It is perhaps surprising that Faraday did not examine the effect of an electric current on his gold sols. Had he done so, he would have discovered the one additional factor which in due course provided the clue to many of their properties, namely that colloidal particles in an aqueous medium (except under special circumstances) move under the influence of an electric field.
The phenomenon of electrophoresis shows that colloidal particles usually carry an electric charge, which in the case of gold sols is negative. The discussion the origin of these charges and the factors that determine their sign is beyond the scope of this brief introduction. For the moment it is sufficient to know that they exist.
This brief account of the properties of one typical colloidal dispersion we are now in a position to examine in the next section the factors that are responsible for the stability of dispersions.
Scottish inorganic and physical chemist. He was born on December 21, 1805, and died on September 16, 1869. He was the founder of Colloid Chemistry and one of the chief founders of physical chemistry. He became professor of chemistry at Anderson's College in Glasgow (1830-37) and at University College, London (1837-54), and finally Master of the Mint (1854-69). From 1828-1833 he studied diffusion of gases and in 1833 proposed Graham's Law, which states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight.
In 1833, Graham distinguished the three phosphoric acids (ortho, pyro, and meta), and his discovery of their polybasicity led Justus von LIEBIG to his modern concept of polybasic acids. He studied the solubility of salts (1827), the absorption of salts by charcoal (1830), and heats of reaction (1841-45). In his work on Colloids (1849-61) he distinguished between crystalloids and colloids as different states of matter and devised a method for their dialysis (separation) by osmosis (both of these terms were coined by Graham).
In the years around 1860 Thomas Graham found that substances such as glue, gelatin, albumin, and starch diffuse very slowly in solution, their diffusion rates being as small. Graham also found that substances of these two types differ markedly in their ability to pass through a membrane such as parchment paper or collodion. If a solution of sugar and glue is put into a collodion bag and the bag is placed in a stream of running water, the sugar soon dialyzes through the bag into the water, and the glue remains behind. This process of dialysis gives a useful method of separating substances of these two kinds. We now recognize that these differences in ability to pass through pores of a membrane and in rates of diffusion are due to differences in size of the solute molecules. Graham thought that there was a deeper difference between ordinary, easily crystallizable substances and the slowly diffusing, nondialysing substances, which he was unable to crystallize.
He named the substance of the latter class COLLOIDS (Greek kolla, glue) in contradistinction to ordinary crystalloids. It is now known that there is not a sharp line of demarcation between the two classes (many substances of large molecular weight have been crystallized). But it has been found convenient to retain the name "colloid" for substances of large molecular weight. Some colloids consist of well-defined molecules, with constant molecular weight and definite molecular shape, permitting them to be ordered in a crystalline array. Proteins have molecular weights ranging from about 10,000 to several hundred thousand.
Graham introduced the words SOL for a colloidal solution (a
dispersion of a solid substance in a fluid medium) and GEL for a dispersion
that has developed a structure that prevents it from
being mobile. A solution of gelatin in water at high temperatures is a SOL and
at low temperatures a GEL. A hydrosol is a dispersion of a solid in
water, and an aerosol is a dispersion of a solid or a liquid substance
in air.
English physicist and chemist, a discoverer of electromagnetic induction. A unit of electricity or an electrical charge that is used in electrolysis and in electronics and which is equal to 96,500 Coulombs is named in his honor.
In 1821 Michael Faraday discovered the fundamentals of electromagnetic rotation.
Mr. Faraday was born Sept. 22, 1791 and died on Aug. 25,
1867. He is known for his pioneering experiments in electricity and magnetism.
Many consider him the greatest experimentalist who
ever lived. Several concepts that he derived directly from experiments, such as
lines of magnetic force, have become common ideas in modern physics.
Faraday was born at Newington, Surrey, near London. He received little more than a primary education, and at the age of 14 he was apprenticed to a bookbinder. There he became interested in the physical and chemical works of the time. After hearing a lecture by the famous chemist Humphry Davy, he sent Davy the notes he had made of his lectures. As a result Faraday was appointed, at the age of 21, assistant to Davy in the laboratory of the Royal Institution in London.
During the initial years of his scientific work, Faraday occupied himself mainly with chemical problems. He discovered two new chlorides of carbon and succeeded in liquefying chlorine and other gases.
He isolated benzene in 1825, the year in which he was appointed director of the laboratory.
Davy, who had the greatest influence on Faraday's thinking, had shown in 1807 that the metals sodium and potassium could be precipitated from their compounds by an electric current, a process known as Electrolysis. Faraday's vigorous pursuit of these experiments led in 1834 to what became known as Faraday's laws of electrolysis.
Faraday's research into electricity and electrolysis was guided by the belief that electricity is only one of the many manifestations of the unified forces of nature, which include heat, light, magnetism, and chemical affinity. Although this idea erroneous, it led him into the field of electromagnetism, which was still in its infancy. In 1785, Charles Coulomb had been the first to demonstrate the manner in which electric charges repel one another, and it was not until 1820 that Hans Christian Oersted and Andre Marie Ampere discovered that an electric current produces a magnetic field. Faraday's ideas about conservation of energy led him to believe that since an electric current could cause a magnetic field, a magnetic field should be able to produce an electric current. He demonstrated this principle of INDUCTION in 1831. Faraday expressed the electric current induced in the wire in terms of the number of lines of force that are cut by the wire. The principle of induction was a landmark in applied science, for it made possible the dynamo, or GENERATOR, which produces electricity by mechanical means.
Faraday's introduction of the concept of lines of force was rejected by most of the mathematical physicists of Europe, since they assumed that electric charges attract and repel one another, by action at a distance, making such lines unnecessary. Faraday had demonstrated the phenomenon of electromagnetism in a series of experiments, however. This experimental necessity probably led the physicist James Clerk Maxwell to accept the concept of lines of force and put Faraday's ideas into mathematical form, thus giving birth to modern field theory.
Faraday's discovery (1845) that an intense magnetic field can rotate the plane of polarized light is known today as a Faraday effect. The phenomenon has been used to elucidate molecular structure and has yielded information about galactic magnetic fields.
Inorganic sols may be made by dispersing a solid substance that is normally
insoluble, such as gold, ferric oxide, and arsenious sulfide, in water. Gold
sols, made by adding a reducing agent to a dilute solution of gold chloride,
were known to the alchemists of the seventeenth century and were studied by
Michael Faraday. They often have striking colors; ruby red,
blue, green, and others that are the result of diffraction of light by the gold
sol particles with dimensions approaching the wavelengths of light. The sols
are stabilized by the presence of electric charge on the surface of the
particles, a negative charge in the case of gold sols. Faraday
found that on addition of a small amount of a salt the ruby gold Sols turned
blue. This is the result of the aggregation of smaller particles to form larger
ones, which scatter light of longer wavelengths. With more salt the particles
coagulate. The coagulation results from the action of small ions with opposite
charge (Na+, Mg++, Al+++) attaching themselves to the negative charges on the
surface of the gold particles and neutralizing the charge. This permits the
particles to approach closely enough to form the coagulum. The coagulating
power of the cations is approximately proportional to the sixth power of their
charge. An aluminum salt is effective as a coagulant at a
concentration 700 times less than a sodium salt.
Faraday described his numerous experiments in electricity and electromagnetism in three volumes entitled Experimental Researches in Electricity (1839, 1844, 1855). Faraday ceased research work in 1855 because of declining mental powers, but he continued as a lecturer until 1861. A series of six children's lectures published in 1860 as The Chemical History of a Candle, has become a classic of science literature.
English physicist, science lecturer, and writer; born in Ireland. He was professor (1853-87) and superintendent (1867-87) at the Royal Institution, London. He investigated light, sound, and radiant heat and studied Alpine glaciers. The scattering of light by Colloids, known as Tyndall Effect , is named after him.
The Austrian chemist born on April 1, 1865 and died September 24, 1929. In Germany in 1903 in collaboration with the physicist H. Siedentopf, he developed the Ultramicroscope. This device allowed him to carry on a research of colloids. Ultramicroscope was important for the examination of colloids.
Zisgmondy studied colored and turbid glasses, and invented the famous Jena milk glass.
In 1925 Zsigmondy was awarded the Nobel Prize for Chemistry for his research on colloids that was titled: "Elucidation of the Heterogeneous Nature of Colloidal Solutions".
American chemist and inventor. Acheson was experimenting with coke and clay in an electric smelting furnace when he noticed that a layer of crystals had formed around the coke core. When this layer was crushed and purified, the resulting crystal grains proved to be nearly as hard as diamonds. They far surpassed all of the natural abrasives such as garnet, pumice, or hard sandstone. The new abrasive was pressed into grindstones, attached to paper or cloth, or used as a polishing powder, and its trade name, Carborundum , eventually entered the English language as a synonym for abrasive. Carborundum is the name given to silicon carbide, a synthesized abrasive discovered in 1891. Like industrial-grade diamond crystals, Carborundum is widely used in industry, although newer artificial crystals such as Borazon have partly supplanted it.
Around the turn of the century Acheson's pioneering work on dispersions of graphite in oil and graphite in water, formed the basis for the Acheson Oildag Company, which was established in 1908. Later, among many other innovations, he invented a process to manufacture synthetic graphite. Acheson is responsible for many developments in the field of colloid chemistry.
During the early 1900's, Acheson pioneered the concept of Lubrication Beyond Oil® with Oildag® and Aquadag® products based on synthetic graphite for superior lubrication. One of the first commercial products introduced by Acheson was graphite-in-oil colloid named Oildag®. It was discovered that when Oildag® was diluted with oil, it provided to be an excellent lubricant and also a release agent for forging operations.
Italian chemist from Milano who later immigrated (1947) to Canada. Pioneer in SYN-SOL Lubrication on which he started to work in 1944 in Germany while working for I.G. Farben and developing lubricants for new jet engines. He experimented with colloidal lubricants in automotive applications since 1953. He developed "The Original Syn!" SYN-SOL lubricant in 1966, while working for a Texaco and developing jet engine lubricants to be used in passenger Jet engines made by General Electric for use in Boeing aircraft. However Texaco had little interest in this very expensive technology, so he later founded SynLube Company in 1969 in Vancouver B.C. Canada to produce and market it. It has been available in the USA since 1985.
SynLube Incorporated currently sells latest version of this colloidal technology under the brand name Lube‑4‑Life®